Overall Score4
- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
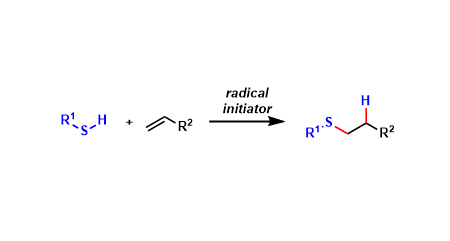
General Characteristics
Thiols and alkenes undergo free-radical mediated S-C bond formation known as thiol-ene reaction. Thanks to its high chemoselectivity and functional group tolerance, it is considered one of the “click chemistry” reactions.
-
General References
- Posner, T. Chem. Ber. 1905, 38, 646.
- Hoyle, C. E.; Bowman, C. N. Angew. Chem. Int. Ed.2010, 49, 1540. DOI: 10.1002/anie.200903924
-
Reaction Mechanism
The thiyl radical formed by radical initiator reacts with the alkene.

-
Examples
Thiol-ene reaction is a promising strategy particularly for the preparation of dendrimers. An example is shown.[1]

-
Experimental Tips
-
References
[1] Killops, K. L.; Campos, L. M.; Hawker, C. J. J. Am. Chem. Soc. 2008, 130, 5062. DOI: 10.1021/ja8006325
-
Related Reactions
-
Related Books
[amazonjs asin=”0470699701″ locale=”US” title=”Click Chemistry for Biotechnology and Materials Science”]
-
External Links