- Generality
- Importance in Chemical Biology
- Future Potential
- Criteria #4
- Criteria #5
-
General Characteristics
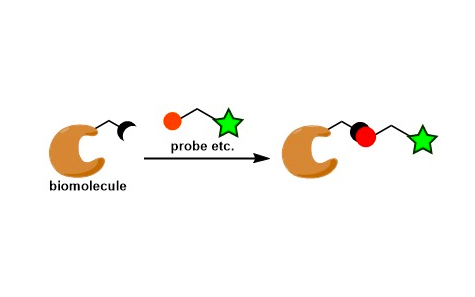
Bioconjugation is a process of covalently functionalizing biological molecules. It is most relevant to protein chemistry and is one of the newest topics in reaction development.
The conjugation of biological molecules with artificial molecules is a particularly important methodology in chemical biology as it helps understand and control specific biological functions.
Bioconjugation is divided into two types depending on whether it targets non-native or native functional groups. The latter has a wider scope of application but is more challenging in terms of controlling reactivity and selectivity. The reactions that can be used are limited as they must be compatible with biological conditions (neutral pH, room temperature, aqueous conditions, etc.) and the reagents and wastes must not be toxic.
It is an essential technology for the preparation of antibody-drug conjugates (ADCs), which are promising next generation therapeutics.
-
General References
<Reviews>
・Prescher, J. A.; Bertozzi, C. R. Nat. Chem. Biol. 2005, 1, 13. doi:10.1038/nchembio0605-13
・Antos, J. M.; Francis, M. B. Curr. Opin. Chem. Biol. 2006, 10, 253. doi:10.1016/j.cbpa.2006.04.009
・ Hackenberger, C. P. R.; Schwarzer, D. Angew. Chem. Int. Ed. 2008, 47, 10030. DOI: 10.1002/anie.200801313
・ Chalker, J. M.; Bernardes, G. J. L.; Lin, Y. A.; Davis, B. G. Chem. Asian J. 2009, 4, 630. DOI: 10.1002/asia.200800427
・Heal, W. P.; Tate, E. W. Org. Biomol. Chem. 2010, 8, 731. DOI: 10.1039/B917894E
・Boyce, M.; Bertozzi, C. R. Nat. Methods 2011, 8, 638. doi:10.1038/nmeth.1657
・Stephanopouuls, N.; Francis, M. B. Nat. Chem. Biol. 2011, 7, 876. doi:10.1038/nchembio.720
・ Hao, Z.; Hong, S.; Chen, S.; Chen, P. R. Acc. Chem. Res. 2011, 44, 742. DOI: 10.1021/ar200067r
・ Witus, L. S.; Francis, M. B. Acc. Chem. Res. 2011, 44, 774. DOI: 10.1021/ar2001292
・ Takaoka, Y.; Ojida, A.; Hamachi, I. Angew. Chem. Int. Ed. 2013, 52, 4088. DOI:
10.1002/anie.201207089
・ Ramil, C. p.; Lin, Q. Chem. Commun. 2013, 49, 11007. DOI:
10.1039/C3CC44272A
・ King, M.; Wagner, A. Bioconjugate Chem. 2014, 25, 825. doi:10.1021/bc500028d
・ Spicer, C. D.; Davies, B. G. Nat. Commun. 2014, 5, 4740. DOI: 10.1038/ncomms5740
・Patterson, D. M.; Nazarova, L. A.; Prescher, J. A. ACS Chem. Biol. 2014, 9, 592. doi:10.1021/cb400828a
・ Shih, H.-W.; Kamber, D. N.; Prescher, J. A. Curr. Opin. Chem. Biol. 2014, 21, 103. DOI:
10.1016/j.cbpa.2014.07.002
-
Reaction Mechanism
-
Examples
- Targeting non-native functional groups
Reaction such as the click reaction (the Huisgen cycloaddition), the Staudinger-Bertozzi ligation, the Suzuki-Miyaura and the Sonogashira coupling reactions, the inverse electron demand Diels-Alder reaction, and olefin metathesis have been used to target non-native functional groups taking advantage of the fact that biological systems do not contain many C-C multiple bonds. Keto-acids and acyl trifluoroborates are also used in recent researches. Because the targeted functional groups have to be incorporated in the biological molecule beforehand, it is difficult to use these reactions in completely natural environment.

- Targeting native functional groups
Typical examples include acylation of lysine, the Michael addition and alkylation of cysteine, and electrophilic aromatic substitution of tyrosine. Native chemical ligation is also a possibility when the N-terminus is cysteine. If ketone or aldehyde group can be pre-installed by selective oxidation, oxime and hydrazone formation can be done as well.

-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Reactions
-
Related Books
[amazonjs asin=”1617791504″ locale=”US” title=”Bioconjugation Protocols: Strategies and Methods (Methods in Molecular Biology)”]
[amazonjs asin=”1588290980″ locale=”US” title=”Bioconjugation Protocols: Strategies and Methods (Methods in Molecular Biology)”]
[amazonjs asin=”0123822394″ locale=”US” title=”Bioconjugate Techniques, Third Edition”]
-
External Links
- Bioconjugation – Wikipedia
- Bioconjugation by Native Chemical Tagging of C -H Bonds (ChemASAP)
- ACS Bioconjugate Chemistry Journal