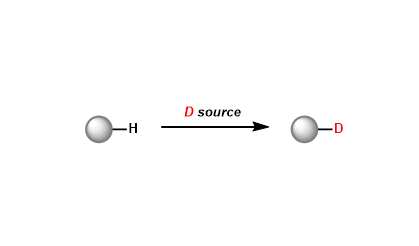
- Reagent Availability
- Importance as Chemical Probe
- Criteria #4
- Criteria #5
-
General Characteristics
The exchange of hydrogen with its isotope deuterium has a wide range of useful applications. The underlying principle is that while replacement of hydrogen with deuterium minimally changes the basic functions of the compound, it gives rise to clear kinetic isotope effect (KIE).
The applications include study of reaction mechanisms, isotope dilution analysis, and structural analysis of biological components. In recent years, deuterated drugs designed to slow down metabolism have been developed and tested in clinical trials. The incorporation of deuterium is also known in the field of optical and electronic devices, where it is used to enhance optical properties and durability.
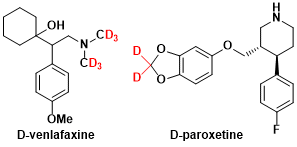
-
General References
<Reviews>
- Junk, T.; Catallo, W. J. Chem. Soc. Rev. 1997, 26, 401. DOI: 10.1039/CS9972600401
- 江嵜 啓祥, 栗田 貴教, 藤原 佑太, 前川 智弘, 門口 泰也, 佐治木弘尚 有機合成化学協会誌2007, 65, 1179. DOI: 10.5059/yukigoseikyokaishi.65.1179
- Atzrodt, J.; Derdau, V.; Fey, T.; Zimmermann, J. Angew. Chem. Int. Ed.2007, 46, 7744. DOI: 10.1002/anie.200700039
- Atzrodt, J.; Derdau, V. J. Label Compd. Radiopharm.2010, 53, 674. DOI: 10.1002/jlcr.1818
- Sawama, Y.; Monguchi, Y.: Sajiki, H. Synlett2012, 23, 959. DOI: 10.1055/s-0031-1289696
- 佐治木弘尚, 薬学雑誌2013, 133, 1177. doi:10.1248/yakushi.13-00218
<H/D-Isotope effect in mechanistic studies>
- Wiberg, K. B. Chem. Rev. 1955, 55, 713. DOI: 10.1021/cr50004a004
- Jones, W. D. Acc. Chem. Res.2003, 36, 140. DOI: 10.1021/ar020148i
- Gallego, M. G.; Sierra, M. A. Chem. Rev.2011, 111, 4857. DOI: 10.1021/cr100436k
- Simmons, E. M.; Hartwig, J. F. Angew. Chem. Int. Ed.2012, 51, 3066. DOI: 10.1002/anie.201107334
-
Reaction Mechanism
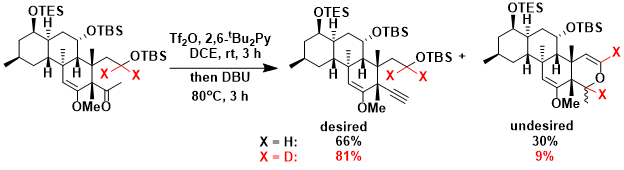
The synthesis of norzoanthamine[1]: When the hydride shift led to the formation of undesired product, deuteration helped increase the yield of desired alkyne product.
Catalytic H/D exchange in deuterium oxide[2]: Normally unreactive C-H bonds can be converted to C-D bonds.
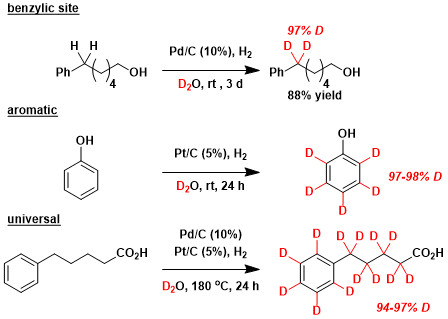
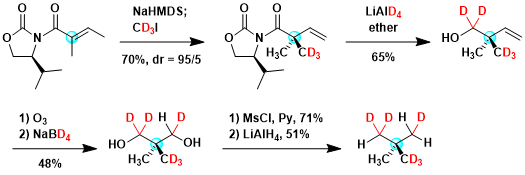
The synthesis of deuterium-labeled optically active neo-pentane[3]: This is a great example in which readily available deuteration reagents are used to prepare an interesting chiral hydrocarbon.
-
Examples
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Miyashita, M.; Sasaki, M.; Hattori, I.; Sakai, M.; Tanino, K. Science 2004, 305, 495. DOI:10.1126/science.1098851
[2] 総説:(a) 江嵜 啓祥, 栗田 貴教, 藤原 佑太, 前川 智弘, 門口 泰也, 佐治木弘尚 有機合成化学協会誌 2007, 65, 1179. DOI: 10.5059/yukigoseikyokaishi.65.1179 (b) Sawama, Y.; Monguchi, Y.: Sajiki, H. Synlett 2012, 23, 959. DOI: 10.1055/s-0031-1289696 (c) 佐治木弘尚, 薬学雑誌 2013, 133, 1177. doi:10.1248/yakushi.13-00218
[3] Haesler, J.; Schindelholz, I.; Riguet, E.; Bochet, C. G.; Hug, W. Nature 2007, 446, 526. doi:10.1038/nature05653
-
Related Reactions
-
Related Books
-
External Links
- Applications and Synthesis of Deuterium-Labeled Compounds (PDF, MacMillan Group)
- Big interest in heavy drugs
- 岐阜薬科大学 佐治木研究室 (The Sajiki Laboratory, Gifu Pharmaceutical University)