- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
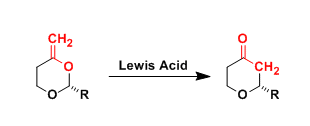
The Lewis acid-promoted rearrangement of enol acetals to cyclic ethers, which involves an oxygen-to-carbon transposition, is known as the Petasis-Ferrier rearrangement. This reaction is generally referred to as the “Type II” Ferrier rearrangement.
Al(III), Hg(II), and Pd(II) reagents are often used for this reaction.
-
General References
・Ferrier, R. J. J. Chem. Soc. Perkin Trans. 1 1979, 1455. DOI: 10.1039/P19790001455
・Petasis, N. A.; Lu, S.-P. J. Am. Chem. Soc. 1995, 117, 6394. DOI: 10.1021/ja00128a044
・Petasis, N. A.; Lu, S.-P. Tetrahedron Lett. 1996, 37, 141. doi:10.1016/0040-4039(95)02114-0
・Ferrier, R. J.; Middleton, S. Chem. Rev. 1993, 93, 2779. DOI: 10.1021/cr00024a008
-
Reaction Mechanism
Note the difference between this reaction and the Type I Ferrier reaction, which is the allylic substitution of 1,2-glycals.

-
Examples
An application in the total synthesis of phorboxazole A.[1]


An interesting variant which effects one-carbon homologation.[2]

-
Experimental Procedure
-
Experimental Tips
The exo-olefin enol acetals are commonly prepared from β-hydroxycarboxylic acids and aldehydes, which form cyclic acetals, followed by olefination with the Petasis reagent.

-
References
[1] Smith, A. B., III; Minbiole, K. P.; Verhoest, P. R.; Schelhaas, M. J. Am. Chem. Soc. 2001, 123, 10942. DOI: 10.1021/ja011604l
[2] O’Neil, E.; Kingree, S. V.; Minbiole, K. P. C. Org. Lett. 2005, 7, 515. DOI: 10.1021/ol047426t
-
Related Reactions
-
Related Books
-
External Links