- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
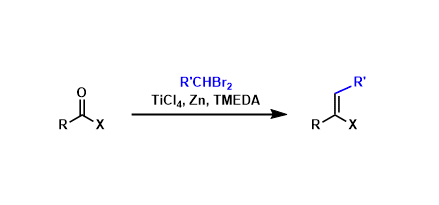
In the Takai olefination, carbonyl compounds are olefinated using organotitanium reagents derived from gem-dihaloalkanes.
Whereas most of other olefination reactions are effective only for aldehydes and ketones, the Takai conditions allow for the olefination of esters and amides too. Thanks to the low basicity of the system, enolizable substrates can be olefinated.
Another feature of this reaction is that while similar reagents such as the Tebbe and the Petasis reagents are limited to methylenation, the Takai reagent can be used to synthesize substituted alkenes. The reaction is generally Z-selective.
-
General References
Takai, K.; Hotta, Y.; Ohshima, K.; Nozaki, H. Tetrahedron Lett. 1978, 19, 2417. doi:10.1016/S0040-4039(01)94789-6
Lombardo, L. Tetrahedron Lett.1982, 23, 4293. doi:10.1016/S0040-4039(00)88728-6
Okazoe, T.; Takai, K.; Oshima, K.; Utimoto, K. J. Org. Chem.1987, 52, 4410. DOI: 10.1021/jo00228a055
Lombardo, L. Org. Synth.1987, 65, 81. [PDF]
Takai, K.; Kakiuchi, T.; Kataoka, Y.; Uchimoto, K. J. Org. Chem.1994, 59, 2668. DOI: 10.1021/jo00089a002
Hartley, R. C.; McKiernan, G. J. JCS Perkin Trans. 12002, 2763. doi: 10.1039/B009709H
-
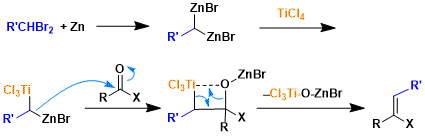
Reaction Mechanism
-
Examples
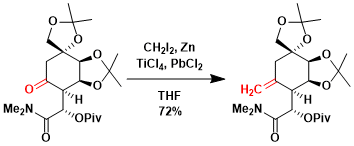
An example in the context of tetrodotoxin synthesis.[1]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Hinman, A.; Du Bois, J. J. Am. Chem. Soc. 2003, 125, 11510. DOI: 10.1021/ja0368305
-
Related Reactions
-
Related Books
[amazonjs asin=”352730634X” locale=”US” title=”Modern Carbonyl Olefination: Methods and Applications”]
-
External Links
Takai-Lombardo Reaktion – Wikipedia(de)