- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
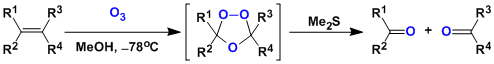
Ozonolysis is a reaction between alkenes and ozone, in which the alkenes are cleaved into a pair of carbonyl compounds.
The reaction is done by introducing ozone gas (generated by silent discharge of oxygen gas) into the solution of substrates in solvents such as dichloromethane and methanol. The reaction tends to be faster in protonated solvents. The reaction works under near neutral conditions.
The products are obtained by decomposing the ozonide intermediate with reducing agents such as zinc, dimethylsulfide, thiourea, and phosphines. The oxidative workup using hydrogen peroxide gives a pair of carboxylic acids, whereas the strongly reductive workup with sodium borohydride gives a pair of alcohols.
-
General References
・Harries, C. Ann. 1905,343, 31.
・Bailey, P. S. Chem. Rev. 1958, 58, 925. DOI: 10.1021/cr50023a005
-
Reaction Mechanism
The “Criegee mechanism” consists of formation of the molozonide by 1,3-dipolar cycloaddition followed by rearrangement to the ozonide. The mechanism is supported by 17O labeling studies. (Ref: Angew. Chem. Int. Ed,. 1975, 87, 745.; Eur. J. Org. Chem. 1998, 1625.)

-
Examples
Example 1.[1]

Ozone can be used to synthesize tertiary alcohols in the presence of silica gel, which adsorbs ozone well at low temperatures. Under similar conditions, amino group can be oxidized to nitro group.

Alkenes in sterically congested positions react slowly.

-
Experimental Procedure
-
Experimental Tips
The reaction mixture turns blue when it is saturated with ozone, so it is easy to tell the endpoint of the reaction.
Ozone gas is toxic. Ozonolysis experiments must be conducted in ventilated hoods.
The handling of ozonide intermediates also requires caution as it is potentially explosive. It is especially unstable in solid form.
-
References
[1] Liu,Z.; Zhang, T. et.al. Tetrahedron Lett. 2001, 42, 275. doi:10.1016/S0040-4039(00)01873-6
-
Related Books