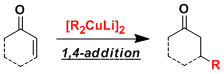- Generality
- Reagent Availability
- Experimental User Friendliness
- Historical Significance
- Criteria #5
-
General Characteristics
The organocuprate reagents [R2CuLi] prepared from copper(I) sources and two equivalents of organolithiums work as strong nucleophiles in 1,4-additions to α,β-unsaturated carbonyl compounds and substitution reactions on sp3 carbons.
These reagents have relatively low basicity, thus tend not to cause unwanted side reactions. Organocuprate and organolithium reagents can be used complementarily since the latter generally react in 1,2-fashion.
Organocuprates are reactive enough to be used for sterically hindered sites. The 1,4-addition reactions can be accelerated by adding hard Lewis acids such as TMSCl.
The Grignard and organozinc reagents can also be used as organometallic reagents in place of organolithiums. The copper(I) sources can be reduced to substoichiometric amounts in these cases.
Two equivalents of the organolithium reagents are essential but only one of them reacts, wasting the other equivalent. The mixed organocuprates ([R(X)CuLi], where X= alkenyl, -CN, -SR’, -NR’2, PR’2, etc.) containing the “dummy ligands” are used to circumvent this problem.
Asymmetric 1,4-addition reactions have been developed recently that use catalytic copper-chiral phosphine complexes.
-
General References
・Modern Organocopper Chemistry, Krause, N. Ed.; Wiley-VCH; 2002.
・Posner, G. H. Org. React. 1972, 19, 1.
・Posner, G. H. Org. React. 1975, 22, 253.
-
Reaction Mechanism
Organocuprates are thought to exist in different structural forms depending on the solvent. Kinetic studies have suggested that the reactions involve the reagent in dimeric form [(R2CuLi)2].
More recently, Nakamura has reported detailed computational studies on the reaction mechanism (Ref: Angew. Chem. Int. Ed. 2000, 39, 3750. 有機合成化学協会誌, 2003, 61, 144.).
In 1,4-addition reactions, the oxidative addition of Cu(I) via the formation of dCu-π*C=C complex generates Cu(III) species. The structure of the Cu(III) has been proposed based on the results of spectroscopic analyses and calculations (Ref: J. Am. Chem. Soc. 2007,129, 7208; J. Am. Chem. Soc.2007, 129, 7210.). These steps and the subsequent reductive elimination are considered as rate-determining.

It had been believed that dummy ligands do not migrate because of their strong affinity to the copper. However, Nakamura’s recent calculation has led to a new theory that the dummy ligands are held in the orientation impossible for migration due to the lithium cation-π interaction.

The mechanism involving Cu(III) intermediates is generally accepted for substitution reactions too.

-
Examples
For cyclic α,β-unsaturated ketones, the stereoselectivity of the addition is greatly influenced by the substrate stereochemistry. The strength of the d-π* complex is the key to achieve high stereoselectivity.


The metal enolates formed after the 1,4-addition can be trapped by various electrophiles to effect three component reactions in one pot. Shown below is an example in the synthesis of prostaglandins.[1]

[R2Cu(CN)Li2] prepared from CuCN as copper source are known as higher order cuprates (the Lipshutz cuprates), which show higher reactivity and distinct chemoselectivity.
![]()
Alkenyl halides and triflates undergo substitution despite the reacting carbons being sp2 hybridized.
![]()
The presence of Lewis acids such as TMSCl and BF3 are known to promote the conjugate addition so that sterically hindered reactants can be used.[2] The enolate intermediates are formed regioselectively.

-
Experimental Procedure

To the stirred solution of epoxide (3.50 g, 40.6 mmol) and CuCN (364 mg, 4.06 mmol) in dry THF (30 mL), was added over 45 min a 1 M solution of vinylmagnesium bromide in THF (52.8 mL, 52.8 mmol) dropwise at -78 °C. The mixture was allowed to warm up to 0 °C, before it was quenched with a saturated NH4Cl solution (20 mL). The layers were separated, the aqueous layer extracted with Et2O (3 x 50 mL), the combined ethereal extracts were washed with brine (20 mL) and dried (MgSO4). Evaporation of the solvent and chromatographic purification of the crude product (silica, Et2O/pentane 1:3) gave the product as pale yellow oil (4.41 g, 95%).[3]
-
Experimental Tips
Organocuprates are thermally unstable and easily decompose through reactions such as homocoupling. The reagents need to be prepared as needed because they tend to be too unstable to be stored.
-
References
[1] Suzuki, M.; Yanagisawa, A.; Noyori, R.J. Am. Chem. Soc. 1988, 110, 4718. DOI: 10.1021/ja00222a033
[2] (a) Yamamoto, Y. Angew. Chem. Int. Ed. 1986, 25, 947. (b) Lipshutz, B. H.; Ellsworth, E. L.; Siahaan, T. J. Am. Chem. Soc. 1989, 111, 1351.
[3]Holub, N.; Neidhorfer, J.; Blechert, S. Org. Lett. 2005, 7, 1227.
-
Related Books
[amazonjs asin=”3527297731″ locale=”US” title=”Modern Organocopper Chemistry”]
[amazonjs asin=”0080370675″ locale=”US” title=”Conjugate Addition Reactions in Organic Synthesis (Tetrahedron Organic Chemistry)”]