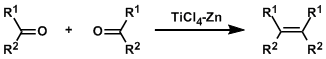- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
In the McMurry reaction, alkenes are synthesized by the reductive coupling of aldehydes and ketones using low valent titanium reagents. This reaction can be thought of as the opposite transformation to ozonolysis. There are many efficient intramolecular examples, including applications to the synthesis of macrocycles and various natural products.
-
General References
・Tyrlik, S.; Wolochowicz, I. Bull. Soc. Chim. Fr. 1973, 2147
・Mukaiyama, T. et al. Chem. Lett. 1973, 1041. doi:10.1246/cl.1973.1041
・McMurry, J. E.; Fleming, M. P. J. Am. Chem. Soc. 1974, 96, 4708. DOI:10.1021/ja00821a076
・McMurry, J. E. Chem. Rev. 1989, 89, 1513. DOI: 10.1021/cr00097a007
・Robertson, G. M. Comp. Org. Syn. 1991, 3, 583.
・Furstner, A.; Jumbam, D. N. Tetrahedron 1992, 48, 5991. doi:10.1016/S0040-4020(01)89848-3
・Lipski, T. A.; Hilfiker, M. A.; Nelson, G. A. J. Org. Chem. 1997, 62, 4566. DOI: 10.1021/jo970792o
-
Reaction Mechanism
The in situ-formed low valent titanium species works as the active reagent. The reaction goes through the pinacol coupling-type intermediates and the driving force is the strong O-Ti bond. The reaction can be stopped at the pinacol intermediates if carried out at low temperature.

-
Examples




The synthesis of 13-hydroxynecembrane.[1]

The McMurry coupling was one of the key steps in the total synthesis of taxol.[2]

-
Experimental Procedure
-
Experimental Tips
-
References
[1] Liu, Z.; Zhang, T.; Li, Y. Tetrahedron Lett. 2001, 42, 275. doi:10.1016/S0040-4039(00)01873-6
[2] Nicolaou, K. C. et al. Nature 1994, 367, 630. doi:10.1038/367630a0
-
Related Books
[amazonjs asin=”352730634X” locale=”US” title=”Modern Carbonyl Olefination: Methods and Applications”]