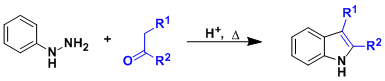- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
Upon heating under acidic conditions, aryl hydrazones synthesized from aldehydes/ketones and aryl hydrazines undergo sigmatropic rearrangement and aromatization to give substituted indole products.
This vintage Fischer indole synthesis is a highly general, standard way to synthesize indole-containing alkaloids and drug molecules.
One of the contemporary modified versions uses the Buchwald-Hartwig coupling to synthesize aryl hydrazones from aryl halides and alkyl hydrazones (the Buchwald modification).

-
General References
・Fischer, E.; Jourdan, F. Ber. 1883, 16, 2241.
・Fischer, E.; Hess, O. Ber. 1884, 17, 559.
・Miyata, O.; Kimura, Y.; Muroya, K.; Hiramatsu, H.; Naito, T. Tetrahedron Lett. 1997, 62, 425. DOI: 10.1016/S0040-4039(99)00583-3
<Buchwald Modification>
・Wagaw, S.; Yang, B. H.; Buchwald, S. L. J. Am. Chem. Soc. 1998, 120, 6621. DOI: 10.1021/ja981045r
・Wagaw, S.; Yang, B. H.; Buchwald, S. L. J. Am. Chem. Soc. 1999, 121, 10251. DOI: 10.1021/ja992077x
<Reviews>
・Van Orden, R. B.; Lindwell, H. G. Chem. Rev. 1942, 30, 69. DOI: 10.1021/cr60095a004
・Robinson, B. Chem. Rev. 1963, 63, 373. DOI: 10.1021/cr60224a003
・Robinson, B. Chem. Rev. 1969, 69, 227. DOI: 10.1021/cr60258a004
・Robinson, B. “The Fischer Indole Synthesis”, John Wiley & Sons Inc., New York, 1982.
・Inman, M.; Moody, C. Chem. Sci. 2012, doi:10.1039/C2SC21185H
-
Reaction Mechanism

-
Examples
The total synthesis of peduncularine.[1]

The synthesis of indometacin.

An application to the complex substrates in the total synthesis of haplophytine.[2]

An interesting example of catalytic asymmetric Fischer indole synthesis.[3]

-
Experimental Procedure
-
Experimental Tips
-
References
[1] Roberson, C. W.; Woerpel, K. A, J. Am. Chem. Soc. 2002, 124, 11342. DOI: 10.1021/ja012152f
[2] Ueda, H.; Satoh, H.; Matsumoto, K.; Sugimoto, K.; Fukuyama, T.; Tokuyama, H. Angew. Chem. Int. Ed. 2009, 48, 7600. doi: 10.1002/anie.200902192
[3] Muller, S.; Webber, M. J.; List, B. J. Am. Chem. Soc. 2011, 133, 18534. DOI: 10.1021/ja2092163
-
Related Books
[amazonjs asin=”0471100099″ locale=”US” title=”The Fischer Indole Synthesis”]