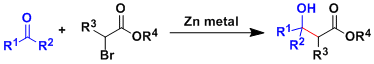- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
In the Reformatsky reaction, the enolates formed from α-haloesters and zinc metal add to aldehydes and ketones.
Zinc enolates are less basic, less reactive, and thus more functional group tolerant than lithium and magnesium counterparts. Esters are generally unreactive as the electrophile.
Metals other than zinc are also known to react in a similar way, such as Sm(II), Cr(II), and Ti(II).
-
General References
・ Reformatsky, S. Ber. 1887, 20, 1210. doi:10.1002/cber.188702001268
・ Reformatsky, S. J. Russ. Phys. Chem. Soc. 1890, 22, 44.
・ Review: Shriner, R. L. Org. React. 1942, 1, 1.
・ Review: Rathke, M. W. Org. React. 1975, 22, 423.
・ Review: Furstner, A. Synthesis 1989, 571. DOI: 10.1055/s-1989-27326
・ Review: Rathke, M. W. Comprehensive Organic Synthesis 1991, 2, 277.
・ Review: Ocampo, R.; Dolbier, W. R. Tetrahedron 2004, 60, 9325. doi:10.1016/j.tet.2004.07.018
-
Reaction Mechanism
The zinc enolates formed in ethereal solvents are known to exist as C-enolate dimers based on spectroscopic and crystallographic analyses. It is considered that the dimers dissociate into monomers and the reaction proceeds with some of the O-enolates reacting through the six-membered cyclic transition state.

-
Examples
An example of intramolecular Reformatsky reaction mediated by SmI2.[1]

-
Experimental Procedure
-
Experimental Tips
-
References
[1] Inoue, M.; Sasaki, M.; Tachibana, K. J. Org. Chem. 1999, 64, 9416. DOI: 10.1021/jo990989b
-
Related Books
[amazonjs asin=”3527298711″ locale=”US” title=”Modern Carbonyl Chemistry”]