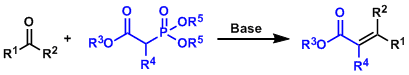- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
-The reaction between a phosphonate ylide and an aldehyde, which proceeds via mechanistic path similar to the Wittig reaction to give an α,β-unsaturated ester.
-The phosphonate substrate can be synthesized by a phospite and an α-haloester, a process known as the Michaelis-Arbuzov reaction. Nitrile, aryl, vinyl, sulfide, amine, and ether groups are also compatible besides ester.
-The reaction is generally E-selective, but there are Z-selective cases depending on the reagent as shown in the example section below.
-The phosphate byproduct is water soluble and easy to remove by simple extraction, which is an important advantage over conventional Wittig reaction.
-
General References
・Horner, L.; Hoffmann, H. M. R.; Wippel, H. G. Ber. 1958, 91, 61.
・Horner, L.; Hoffmann, H. M. R.; Wippel, H. G.; Klahre, G. Ber. 1959, 92, 2499.
・Wadsworth, W. S., Jr.; Emmons, W. D. J. Am. Chem. Soc. 1961, 83, 1733. doi:10.1021/ja01468a042
・Boutagy, J.; Thomas, R. Chem. Rev. 1974, 74, 87. doi:10.1021/cr60287a005
・Wadsworth, W. S., Jr. Org. React. 1977, 25, 73.
・Maryanoff, B. E.; Reitz, A. B. Chem. Rev. 1989, 89, 863. doi:10.1021/cr00094a007
・Kelly, S. E. Comprehensive Organic Synthesis 1991, 1, 729.
-
Reaction Mechanism

-
Examples
When the phosphonate is (CF3CH2O)2P(O), Z-product is formed preferentially (the Still-Gennari modification).[1]

Similarly, (ArO)2P(O) also renders the reaction Z-selective (the Ando modification).[2]
 Using LiCl as an additive, the reaction can proceed with a weaker base such as DBU and Hünig’s base (the Roush-Masamune conditions). This is useful when the reactants are unstable under strongly basic conditions involving NaH or LDA. LiCl is considered to act as a Lewis acid to increase the acidity of the activated proton.
Using LiCl as an additive, the reaction can proceed with a weaker base such as DBU and Hünig’s base (the Roush-Masamune conditions). This is useful when the reactants are unstable under strongly basic conditions involving NaH or LDA. LiCl is considered to act as a Lewis acid to increase the acidity of the activated proton.

-
Experimental Procedure
-
Experimental Tips
-
General References
[1] Still, W. C.; Gennari, C.Tetrahedron Lett., 1983, 24, 4405.
[2] (a) Ando, K. J. Org. Chem. 1997, 62, 1934. DOI: 10.1021/jo970057c (b) Ando, K. J. Org. Chem. 1998, 63, 8411. DOI: 10.1021/jo981337a (c) Ando, K. J. Org. Chem. 1999, 64, 6815. DOI: 10.1021/jo9909150
[3] Blanchette, M. A.; Choy, W.; Davis, J. T.; Essenfeld, A. P.; Masamune, S.; Roush, W. R.; Sakai, T. Tetrahedron Lett. 1984, 25, 2183.
-
Related Books
[amazonjs asin=”352730634X” locale=”US” title=”Modern Carbonyl Olefination: Methods and Applications”]