Y, Sun.; J, Liu.; H, Zhang.; Y, Huo.; X, Lv.; Y, Shi.; W, Guo.; J. Am. Chem. Soc., 2014, ASAP
DOI: 10.1021/ja504156a
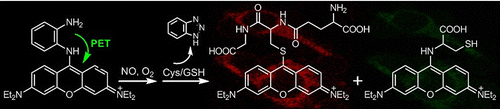
A mitochondria-specific fluorescent probe for NO (1) was synthesized by the direct conjugation of a pyronin dye with one of the amino groups of o- phenylenediamino (OPD). The probe could selectively detect NO over dehydroascorbic acid (DHA), ascorbic acid (AA), and methylglyoxal (MGO) as well as the reactive oxygen/nitrogen species (ROS/RNS) with the significant off−on response due to the production of a red- emission triazole 2. In the presence of cysteine/glutathione (Cys/GSH), 2 could be further transformed into a green- emission aminopyronin 4 and a red-emission thiopyronin 5, respectively. Assisted by intracellular Cys and GSH, the probe demonstrated its potential to monitor mitochondrial NO in a dual-channel mode.
Introduction
Organism often produces NO molecule as result of various reactions. Therefore, to make it visible provide us with lot of variable information in organism. In the article, Wei Guo group at Shanxi University took advantage of classical strategyies[1] for detecting NO molecule and prepared mitochondria targeted dual-color switchable NO sensitizer.
Molecular design
Traditional strategy for off-on fluorescence detection of NO relies on in-situ formation of benzotriazole which is responsible for subsequent PET. As detailed in the article, the presence of benzotriazole sometime causes some undesired effects (e.g. pH change negates off-on fluorescence switch and addition of poisonous reagents is needed). In this work, one amine in o-phenylenediamine was directly attached to the fluorophore so that subsequent formed benzotriazole can be less sensitive to surrounding environment.
In vitro and in vivo experiment
In addition to stable fluorescent detection of NO, fluophores in the red bright state can further react with glutathione (GSH) [2]accompanied by the change in emission color to green. As well as the high selectivity to NO molecule in vitro experiment, the prepared sensitizer function in live imaging. After incubation, the dyes localized to mitochondria, which was confirmed by Mitotracker. The changes in emission from dirk to red and from red to green were also observed in live cell.
Reference
[1]”Fluorogenic and Chromogenic Rhodamine Spirolactam Based Probe for Nitric Oxide by Spiro Ring Opening Reaction”
Zheng, H.; Shang, G.-Q.; Yang, S.-Y.; Gao, X.; Xu, J.-G. Org. Lett. 2008, 10, 2357. DOI: 10.1021/ol800206x
A new fluorogenic and chromogenic probe rhodamine B spirolactam (1) was developed that in aqueous solutions exhibited a highly selective and sensitive “turn-on” type spectral response toward NO detection following a NO-induced spiro-ring opening reaction in 1.
[2]”Simultaneous fluorescent imaging of Cys/Hcy and GSH from different emission channels”
Liu, J.; Sun, Y.-Q.; Zhang, H.; Huo, Y.; Shi, Y.; Guo, W.Chem. Sci. 2014, 5, 3183.
DOI: 10.1039/C4SC00838C
A 4-methoxythiophenol-substituted pyronin dye 1 was exploited as reaction-type fluorescent probe for biothiols Cys/Hcy and GSH. The probe itself is nonfluorescent due to the photoinduced electron transfer (PET) process. The Cys (or Hcy)-induced substitution–rearrangement cascade reaction and GSH-induced substitution reaction with the probe lead to the corresponding aminopyronin and thiopyronin dyes with distinct photophysical properties, enabling Cys/Hcy and GSH to be detected from visible and near-infrared (NIR) emission channels, respectively, in pure PB buffer with relatively fast kinetics and obvious fluorescence turn-on response. Assisted by laser scanning confocal microscope, we also demonstrated that probe 1 could simultaneously sense Cys/Hcy and GSH in B16 cells in multicolor imaging.